Managing patient data is the most challenging task in all hospitals, especially in clinical trials. Initially, data collection and sharing were scattered and stored in different systems. The fragmented clinical data made the situation hectic for healthcare providers, researchers, and pharmaceutical companies. While they needed the clinical data, they couldn’t access and utilize the vital information. This inefficiency caused a long delay in the pace of research and stopped timely decisions to improve patient care.
Enter Fusion, a groundbreaking solution within the Helix brand designed to revolutionize clinical trial data sharing. Fusion leverages the HL7 FHIR (Fast Healthcare Interoperability Resources) standard, a modern and widely adopted framework for healthcare data exchange. By enabling seamless integration across various systems, platforms, and stakeholders, Fusion ensures that clinical trial data is not trapped in isolated silos but is shared in real-time, securely, and at scale.
But how does Fusion make a difference? Imagine a scenario where researchers, healthcare providers, and pharmaceutical companies all have access to up-to-date clinical trial data at their fingertips. With Fusion, this becomes a reality. Accessing data in real-time accelerates the research process, helps identify trends faster, and ultimately leads to more informed decisions. Moreover, the integration of HL7 FHIR ensures that data is standardized, reducing inconsistencies and improving the quality of insights derived from the data.
Fusion’s unified approach simplifies data sharing and improves the overall efficiency of clinical trials. By enabling secure, efficient, and scalable data exchange, Fusion plays a pivotal role in accelerating research, reducing time to market for new treatments, and ultimately improving patient outcomes. Ready to see how Fusion can transform your clinical trial processes? Let’s dive deeper into its capabilities.
Table of Contents
Real-time example of challenges in Clinical Trial Data Sharing
In 2020, during the early days of the COVID-19 pandemic, hospitals in the U.S. faced major challenges due to delays in sharing clinical trial data. Many hospitals were part of trials testing new COVID-19 treatments but had trouble accessing real-time data from different trial sites. This made it hard for doctors to make fast decisions about the best care for patients.
For instance, one large hospital in New York was involved in a clinical trial for a new COVID-19 drug. However, due to slow data sharing between the research team and the hospital, doctors couldn’t quickly evaluate how the drug was affecting patients. This delay meant that doctors missed the chance to adjust treatment plans based on the latest data, which could have helped improve patient recovery.
Additionally, the slow flow of information delayed reporting any drug-related side effects, slowing down decisions about whether to continue or change the trial.
This situation showed just how important it is to have fast, reliable systems for sharing clinical trial data. With information stuck in different systems, hospitals struggled to stay updated. The incident is clear: evidence of the need for better tools to share data quickly, helping doctors make the best choices for patient care.
Before diving into challenges, let’s understand clinical trial data.
What is Clinical trial data?
Clinical trial data refers to information gathered during a clinical trial to test the effectiveness and safety of a treatment, drug, or medical device. This encompasses a wide range of measurements, including patient demographics, health conditions, treatment regimens, test results, side effects, and outcomes.
It helps researchers and healthcare providers gauge whether a treatment works as intended and what risks it poses. The data is carefully recorded and analyzed to ensure scientific rigour and regulatory compliance, and it forms the basis for approval by health authorities like the FDA.
Challenges faced by hospitals by clinical trial data:
Some factors further amplify the challenges of clinical trial data sharing that are specific to the healthcare system, regulations, and technology. Some of the key challenges of clinical data sharing for U.S. hospitals include:
1. Fragmented Healthcare System
The healthcare system is extremely siloed, with many private and public entities using different Electronic Health Record (EHR) systems. This makes seamless data sharing between hospitals, healthcare providers, or even research institutions difficult. Different hospitals may use different software; hence, integrating clinical trial data from diverse sources results in incompatibility.
2. Complex Regulatory Environment
Several federal and state regulations must be followed in clinical trial data sharing, such as the Health Insurance Portability and Accountability Act, which has specific rules on protecting patient privacy and data security.
Sharing clinical data between multiple entities, particularly if such data crosses state or national borders, increases the difficulty of achieving HIPAA compliance and can involve more legal complexity. U.S. hospitals also face FDA regulations, which require data shared during clinical trials to meet certain standards, such as data integrity and traceability requirements.
3. Concerns About Data Privacy and Patient Consent
It heightens concern in U.S. hospitals about patient privacy and management of consent, especially due to the growing number of data breaches in the health sector.
While the HIPAA Privacy Rule is intended to protect patient information, it is also challenging when the interpretations of these laws vary across states and institutions. For instance, some patients may not understand their data or know how it will be used or shared in clinical trials.
Maintaining data sharing for clinical trials and ensuring patient privacy remain essential issues, and hospitals require explicit consent from patients before sharing any data.
4. Data Ownership and Control Issues
Within U.S. hospitals, there is often a lack of clarity over who owns the data generated in clinical trials—the hospital, the research institution, or the patient. This ambiguity may help impede data sharing.
The fear of losing intellectual property (IP) and control of the data can deter hospitals from sharing their data with pharmaceutical companies or other researchers. Sharing valuable data might result in competitive disadvantages or conflicts over IP rights.
5. Limited Interoperability Between Systems
This is one of the significant barriers to effective clinical trial data sharing in U.S. healthcare systems due to the lack of universal interoperability standards. Many hospitals have proprietary or outdated systems that are incompatible, thus creating a problem in sharing data seamlessly.
Though significant strides have been taken through policies like the 21st Century Cures Act and standard implementation using Fast Healthcare Interoperability Resources (FHIR), consistent industry-wide adoption and application across this platform remain far from reality.
6. Financial and Resource Constraints
- U.S. hospitals, especially smaller or community-based institutions, may struggle to allocate sufficient financial and human resources for data-sharing infrastructure. These hospitals may not have the funding to invest in IT systems, data governance frameworks, or staff training to manage secure and efficient data sharing.
- Data sharing often involves significant costs for system upgrades, cloud storage, and legal compliance, which can be prohibitive for smaller hospitals operating under tight budgets.
7. Inconsistent Data Quality and Validation
- Hospital-shared clinical trial data may be inconsistent in terms of quality, accuracy, and completeness. This is particularly problematic when hospitals use different data entry protocols, leading to discrepancies in data that can affect research outcomes.
- Hospitals may struggle to ensure data validity due to a lack of standardized data validation processes. This can create issues when sharing clinical trial data across institutions, as discrepancies may go unnoticed unless the data is thoroughly reviewed and cleaned.
8. Pressure from Payers and Insurers
- U.S. hospitals are under increasing pressure from payers and insurers to demonstrate cost-effectiveness and patient outcomes. This often leads to reluctance to share clinical trial data, as hospitals may fear that sharing could expose inefficiencies or raise questions about treatment outcomes.
- Insurance companies may be particularly concerned about the implications of shared data, especially if it reveals discrepancies or suboptimal care, leading to financial risks for the hospitals involved.
9. Legal and Liability Risks
- Data sharing in clinical trials carries legal risks, particularly if there are data breaches, violations of consent agreements, or failure to meet regulatory standards. U.S. hospitals must take significant precautions, including robust contracts, indemnity clauses, and insurance policies, to mitigate the legal risks associated with data sharing.
- Hospitals also face liability issues if the shared data leads to inaccurate research findings or the use of unsafe treatments, which can result in lawsuits or regulatory penalties.
10. Technology-based Security Threats
Health facilities are always the centre for cyberattacks, particularly ransomware, breaches, and other phishing attacks that could breach patient information. This brings upon severe accountability requirements for the hospital regarding these threats over protecting clinical trial data being accessed.
Cyberattacks are becoming more sophisticated, and a hospital needs to continually refresh its cybersecurity infrastructure to prevent emerging threats while still being able to share data in real-time.
11. Lack of Skilled Personnel
Most hospitals face challenges recruiting and retaining staff with specialized skills for managing and sharing clinical trial data, including expertise in data science, bioinformatics, and regulatory compliance. Without the right personnel, data-sharing initiatives can suffer significant delays or inefficiencies as hospitals struggle to manage, validate, and disseminate clinical trial data effectively.
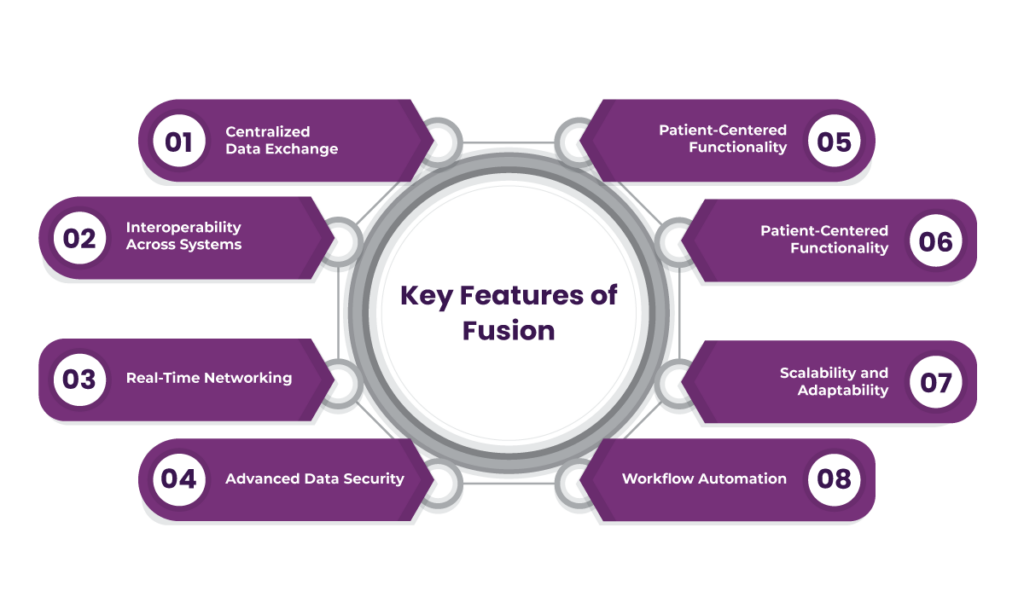
Key Features of Fusion: A Unified FHIR Solution for Clinical Trials
1. Centralized Data Exchange
Fusion brings integration with FHIR by establishing a central data repository referred to as the FHIR Data Server. This server forms a unified space for clinical data standardization, like patient records, medication lists, and care plans, which are shared and retrieved among stakeholders. It makes possible access, retrieval, or modification of information in real-time using RESTful APIs to keep participants’ information current at all times. In addition, Fusion’s system normalizes data from diverse sources into FHIR-compatible formats, which enables seamless communication between previously incompatible systems.
2. Interoperability Across Systems
With Fusion’s cross-platform compatibility, sharing healthcare data will become smooth across multiple systems, including Electronic Health Records, laboratory systems, mobile health applications, and wearable devices. Also, its functionality to map legacy data formats like HL7 v2 or CDA into FHIR resources helps provide backward compatibility for adoption without much disruption to an organization’s current workflows. The integration of SMART on FHIR allows third-party applications to securely access clinical data, thereby enabling the expansion of healthcare systems and improving data sharing between clinical trials.
3. Real-Time Networking
Real-time data exchange is critical in clinical trials, where timely decision-making can mean success or failure. Fusion facilitates the immediate sharing of patient data, including medical histories, lab results, and prescriptions, across healthcare providers, ensuring that all participants in a clinical trial have access to the latest information. This real-time data sharing also improves care coordination, allowing primary care physicians, specialists, and allied health professionals to work well together in clinical trials.
4. Advanced Data Security
When patients’ sensitive data is transferred, Data security is necessary. With Fusion, clinical trial data is safeguarded by applying role-based access control and end-to-end encryption via protocols such as TLS and AES, in addition to managing patient consent. Such data security mechanisms ensure that a firm complies with health-related regulatory measures such as HIPAA and GDPR and ensures the patient’s discretion over data sharing while securing sensitive information.
5. Patient-Centered Functionality
This brings the patient to the centre of clinical trial data sharing. Through a secure app or portal, patients can access their medical records, participate in remote monitoring, and share their health data with clinicians involved in the trial. This empowers patients to take control of their healthcare while enabling real-time collaboration between clinical trial teams and the patients themselves. Besides this, Fusion enables interoperable care plans to be shared with everyone involved in a patient’s care to provide them with the latest information about their treatment.
6. Analytics and Decision Support
Fusion incorporates advanced analytics and decision-support tools to enhance clinical trial outcomes. It aggregates anonymized patient data, providing valuable insights into population health trends to optimise treatment strategies. Moreover, Fusion brings in AI-driven insights to support predictive analytics, diagnostics, and treatment recommendations. Such data-driven approaches improve patient outcomes and smooth out clinical trial processes.
7. Scalability and Adaptability
.As the scale and complexity of clinical trials grow, Fusion’s cloud-based architecture ensures healthcare organizations can scale their infrastructure to meet demands. As it’s built modularly, new functionalities or emerging technology integration becomes possible without affecting existing workflows in clinical trial systems.
8. Workflow Automation
Fusion’s workflow automation features significantly reduce administrative overhead, improving efficiency in clinical trials. For example, Fusion automates data exchanges between clinical trial systems, ensuring that patient records are synchronized between providers without the need for manual data entry. Automated referral processes, appointment reminders, and lab result notifications further streamline clinical trial workflows, allowing trial teams to focus on patient care and research activities.
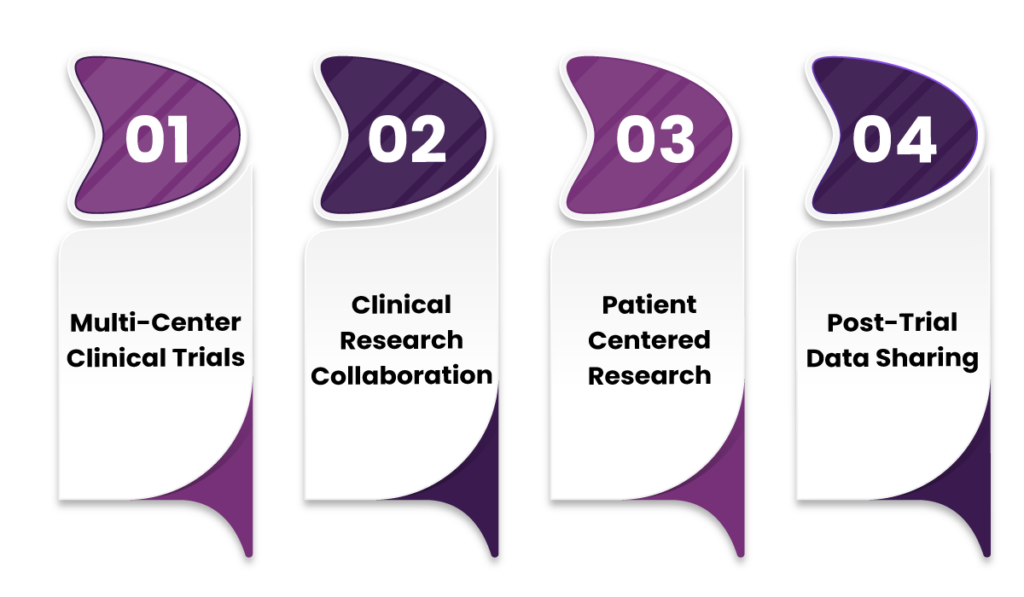
Use Cases for Fusion in Clinical Trial Data Sharing
1. Multi-Center Clinical Trials
In multi-centre clinical trials, patient data is often scattered across multiple sites, creating barriers to efficient data sharing and decision-making. Fusion’s centralized data repository allows trial teams to access all patient data in real time, regardless of where it is stored. This ensures that researchers can make informed decisions based on the most up-to-date information, leading to faster trial completion and better patient outcomes.
2. Clinical Research Collaboration
Clinical research often involves collaboration between multiple stakeholders, such as pharmaceutical companies, research institutions, and healthcare providers. Fusion’s real-time data exchange capabilities enable seamless collaboration between these stakeholders, ensuring that trial participants’ data is shared securely and efficiently. By using FHIR, Fusion ensures that all parties involved can access the same standardized data, reducing errors and improving the overall quality of research.
3. Patient-Centered Research
Involving patients in the research process is crucial to modern clinical trials. Fusion’s patient-centred functionality enables patients to share their health data with clinical trial teams and access their own medical records. This improves patient engagement and ensures that patients are empowered to make informed decisions about their participation in clinical trials. Additionally, Fusion’s integration with wearable devices allows continuous monitoring of patients, enabling researchers to collect real-time data for more accurate results.
4. Post-Trial Data Sharing
Once a clinical trial is completed, the data collected needs to be shared with regulators, researchers, and other stakeholders for analysis and decision-making. Fusion’s interoperability features ensure that post-trial data is easily shared across systems, enabling a seamless transition from trial completion to regulatory review or publication.
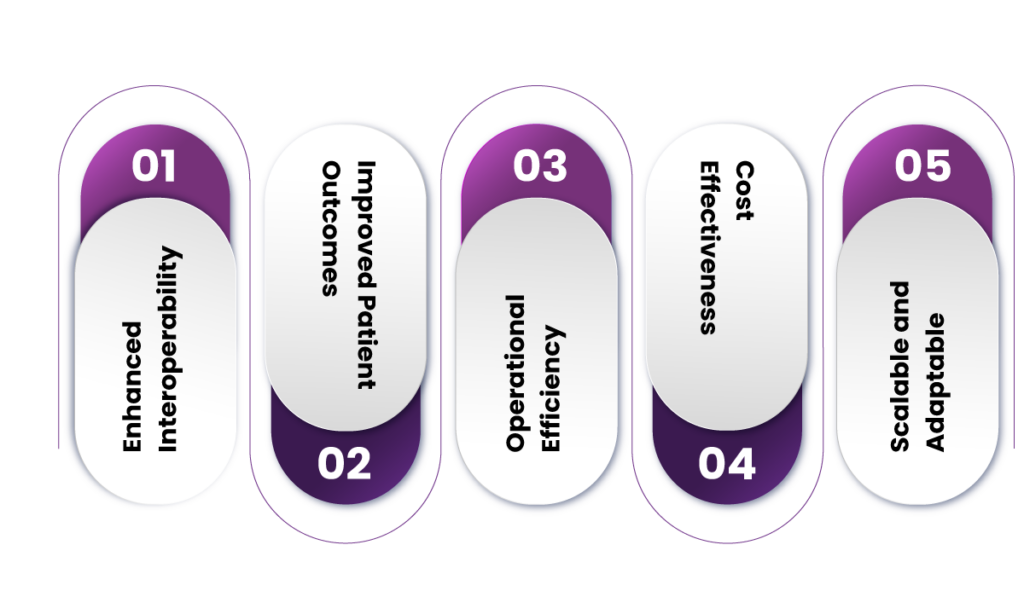
Benefits of Fusion for Clinical Trials
1. Enhanced Interoperability
By providing a unified platform for data exchange, Fusion ensures that clinical trial stakeholders can communicate seamlessly across different systems, improving collaboration and reducing the chances of miscommunication.
2. Improved Patient Outcomes
With real-time access to accurate, up-to-date patient data, clinicians are better equipped to make informed decisions, resulting in improved patient care and better trial outcomes.
3. Operational Efficiency
Fusion automates data exchange, reducing manual processes and administrative burdens. This leads to faster trial execution, reduced costs, and improved efficiency in clinical research.
4. Cost-Effectiveness
By reducing the need for custom integrations and improving data-sharing efficiency, Fusion helps reduce the cost of clinical trials. Additionally, its ability to leverage existing systems and technologies makes it a cost-effective solution for healthcare organizations and clinical researchers.
5. Scalable and Adaptable
Fusion’s cloud-based architecture and modular design ensure that it can scale with the growing demands of clinical trials and adapt to future innovations in healthcare and clinical research.
Challenges and Considerations for Implementing Fusion
While Fusion offers significant advantages in clinical trial data sharing, organisations must address several challenges and considerations before implementation.
1. Governance and Compliance
Healthcare organizations must establish robust governance frameworks to ensure that clinical trial data is shared securely and complies with HIPAA, GDPR, and FDA guidelines. Implementing Fusion will require organizations to review their data-sharing policies and ensure they have the necessary security measures.
2. Integration with Legacy Systems
Many healthcare organizations still rely on legacy systems that may not be fully compatible with FHIR. While Fusion’s data mapping tools can convert legacy data into FHIR resources, integrating these systems may require additional resources and expertise.
3. Training and Adoption
Successful implementation of Fusion requires training of healthcare staff and clinical trial teams on the new system and workflows. Organizations will need to invest in training and support to ensure smooth adoption and maximize the benefits of Fusion.

Conclusion: The Future of Clinical Trial Data Sharing
Fusion’s ability to streamline clinical trial data sharing through the FHIR standard is a game-changer for the healthcare and clinical research industries. By enabling real-time, standardized data exchange across systems, Fusion enhances collaboration, improves patient outcomes, and reduces the costs and complexities associated with clinical trials. As the healthcare industry continues to embrace interoperability and data-driven decision-making, Fusion will play a crucial role in shaping the future of clinical research and patient care.
Frequently asked question
- What is Fusion, and how does it improve clinical trial data sharing?
Fusion is a platform designed to enhance clinical trial data sharing. It leverages the HL7 FHIR standard to ensure real-time, secure, and seamless data exchange across multiple systems and stakeholders.
- How does Fusion ensure data security in clinical trials?
Fusion employs role-based access control, end-to-end encryption (TLS and AES), and compliance with regulations like HIPAA and GDPR to secure patient data during clinical trials.
- Can Fusion integrate with existing hospital systems?
Yes, Fusion is designed for interoperability and can integrate with a wide range of healthcare systems, including Electronic Health Records (EHR), laboratory systems, and mobile health apps.
- What makes Fusion different from other clinical trial data-sharing platforms?
Fusion stands out by offering real-time data exchange, centralized data repositories, and robust analytics. It also adheres to the HL7 FHIR standard to ensure compatibility across diverse platforms.
- How does Fusion handle patient consent for data sharing in clinical trials?
Fusion incorporates patient consent management, allowing patients to control the sharing of their medical data. This ensures compliance with privacy regulations while empowering patients in the decision-making process.
- Can Fusion be used for multi-centre clinical trials?
Yes, Fusion’s centralized data repository allows for the seamless sharing of patient data across multiple trial sites, improving collaboration and decision-making in multi-center trials.
- How does Fusion support real-time data exchange?
Fusion enables real-time data exchange through RESTful APIs, ensuring that clinical trial teams have access to the most current patient data at all times, regardless of where it is stored.
- What is the role of HL7 FHIR in Fusion?
HL7 FHIR is a modern data exchange standard that Fusion leverages to ensure that clinical trial data is consistent, standardized, and easily shareable across different systems and stakeholders.
- How does Fusion help with regulatory compliance?
Fusion ensures compliance with key healthcare regulations, including HIPAA and GDPR, by securing patient data and supporting the necessary privacy and security measures for clinical trial data sharing.
- Can Fusion be scaled for large clinical trials?
Yes, Fusion’s cloud-based architecture allows for scalability, making it suitable for large and complex clinical trials by accommodating increasing data volumes and integrating emerging technologies.
- Does Fusion improve clinical trial outcomes?
Yes, Fusion enhances clinical trial outcomes by providing real-time data access, improving collaboration between stakeholders, and enabling better decision-making through advanced analytics and predictive insights.
- How does Fusion support patient-centred research?
Fusion empowers patients by giving them access to their medical records, enabling participation in remote monitoring, and allowing them to share their health data directly with clinical trial teams.
- What are the key benefits of using Fusion in clinical trials?
Key benefits include faster research timelines, better patient outcomes, real-time data access, improved collaboration, enhanced data security, and reduced administrative overhead.
- How does Fusion handle data from wearable devices?
Fusion integrates with wearable devices to collect continuous patient data, which is then shared in real-time with clinical trial teams to enable more accurate and timely decision-making.
- What types of healthcare organizations can benefit from using Fusion?
Hospitals, research institutions, pharmaceutical companies, and healthcare providers involved in clinical trials can all benefit from using Fusion for more efficient and secure clinical trial data sharing.